Join the BETA test for RiskGATOR
YOU can help shape the future of Lab QC!
Take a Bite Out of Risk!
Your challenge:
|
ISO 15189:2022 requires that you implement risk management, with many specific requirements, but it does not tell you how to do that.
“Although this document requires that the laboratory identifies and addresses risks, there is no requirement for any particular risk management method. “ |
Our solution:
RiskGator Software
The first major advancement in medical laboratory quality management in decades!

The Truth Behind Every Quality Control Process
- QC flags,
- Reagent changes,
- Reruns, and
- Endless, stressful, checklists
- Delays, and
- Unnecessary costs.
Even the most dedicated staff can feel overwhelmed by systems that weren’t built for today’s challenges. Test this revolutionary software and experience cutting edge laboratory quality control evolving into risk management!
Be on the BETA Tester Team to put RiskGATOR through its paces:
As a Beta tester you get FREE access to RiskGATOR for 90 days in exchange for your comments and suggestions.
(ISO 15189:2022)
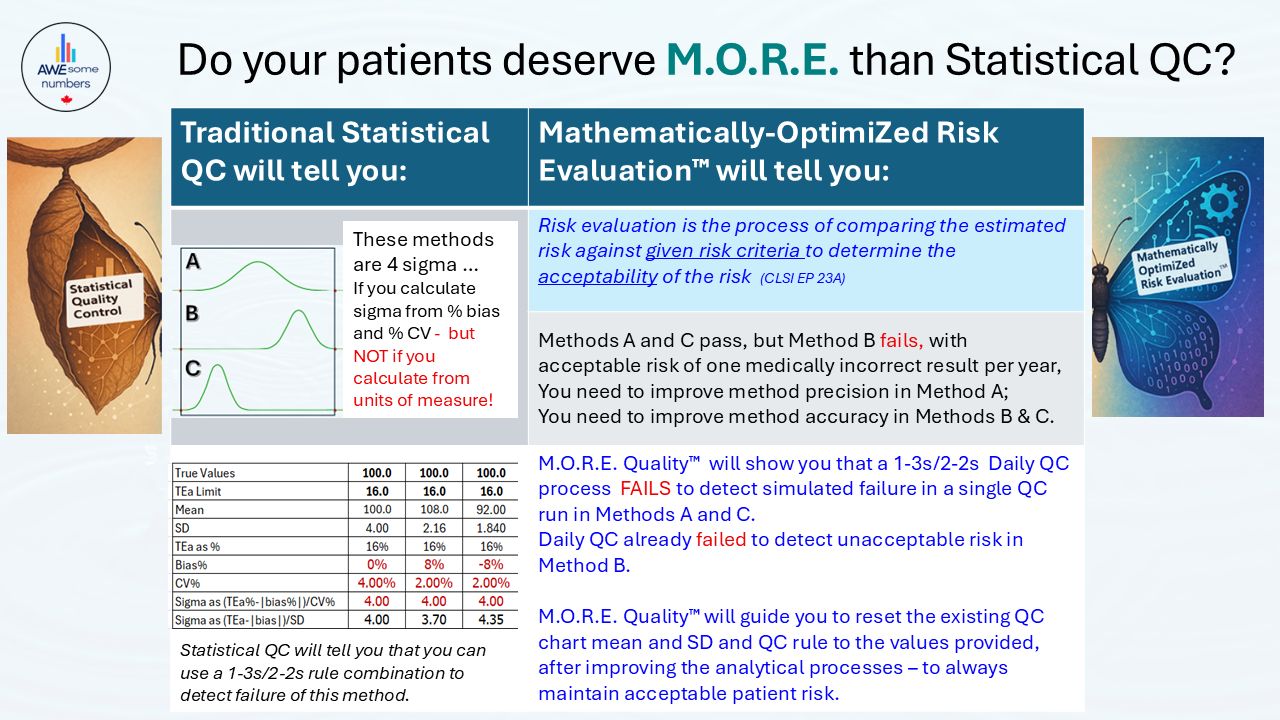
“Enable routine performance of risk evaluation – the process of comparing the estimated risk against given risk criteria to determine the acceptability of the risk”
Meets CLSI recommendation that
“At the least, the ability of the QC procedures to detect medically allowable error should be evaluated”
Allows the lab director to
define the acceptable risk as the number of medically incorrect results (MIRs) per year for each analyte based on clinical need.
Saves time, reduces stress and saves money
within the laboratory by eliminating over 80% of QC flags.
Provides consistent proven guidance
to all staff at all times to investigate and resolve problems – to continuously maintain acceptable risk levels.
Significantly reduces the number and healthcare cost
of medically incorrect results (MIRs) that fail the allowable error limit defined by the lab director.
Compare Traditional Statistical QC to Mathematically-OptimiZed Risk Evaluation™
| Mathematically-OptimiZed Risk Evaluation™ | |
| Defines acceptable performance as a sigma value based on concepts from industry in 1987. • Includes unverified assumptions of shifts over time. • Based on incorrect formula |
RiskGATOR™ software is powered by M.O.R.E. Quality™, the risk management process created by Zoe Brooks. Defines acceptable risk as the number of MIRs per year and per failure event that vary by analyte and clinical setting. |
| Estimates errors per one million results based on sigma value from assorted formulae. |
“Compares the estimated risk against given risk criteria to determine the acceptability of the risk” (CLSI) Quantifies number of medically incorrect results per year at each QC sample level. Estimates the probability of occurrence of harm as the likelihood of an analytical process producing one error every ‘x’ hours, days, weeks, months or years. |
| No breakdown for cause of errors |
Analyses QC data to advise users if an analytical process requires improvement in method accuracy and/or precision. Guides investigation of common faults that cause reduced method accuracy and/or precision. Presents intuitive Risk Distribution Graphs and Risk Evaluation Charts |
| Assumes QC processes are effective. |
Meets CLSI recommendation that “At the least, the ability of the QC procedures to detect medically allowable error should be evaluated” Quantifies risk as the number of Medically Incorrect Results (MIRs) per failure based on simulated failure of an unacceptable number of results and acceptable risk level for daily QC |
| Will not detect failure in a single run when sigma is low |
Guides correction of the Daily QC Process Design to create a process that is verified to detect failure in a single QC run. Provides an interim QC process to increase the number of QC samples tested per run until analytical and QC processes have been improved. Guides assignment of QC chart SD to enable a range of QC rules from 1-2s to 1-3.5s in software that is only capable of a single 1-2s or 1-3s rule |
| Combines Analytical Process Quality and Daily QC Process Effectiveness into a single intuitive Risk-Based Quality Grade that conveys acceptability of risk and recommended action. | |
| Quantifies avoidable healthcare costs caused by lab errors. | |
| Evaluates acceptability of cost of error. | |
| Recommends improvement to reduce costs. | |
| Estimates the costs to perform laboratory QC based on number of tests and false positives. | |
| Reduces false positive QC flags | |
| Guides the lab to “identify and select opportunities for improvement and develop, document, and implement any necessary actions. “ | |
| Guides the lab to “direct improvement activities be at areas of highest priority based on risk assessments and the opportunities identified.” |
Be on the BETA Tester Team to put RiskGATOR through its paces:
As a Beta tester you get FREE access to RiskGATOR for 90 days in exchange for your comments and suggestions.
Every patient deserves results they can trust.
Every lab professional deserves tools that make trust possible.
RiskGATOR is where that trust begins.
Unlock the full potential of your laboratory with RiskGATOR. Our advanced software simplifies complex statistical metrics, providing you with clear, actionable insights. Transform your quality control processes AND be ready for your next inspection!
About AWEsome Numbers Inc.
Founded in 2012 by Zoe Brooks, AWEsome Numbers Inc. is dedicated to revolutionizing clinical laboratory quality control. Our mission is to standardize quality control processes across laboratories, ensuring consistent, reliable, and medically sound results. Our proprietary algorithms and innovative approach set us apart as leaders in the field, committed to advancing laboratory excellence.